The peroxide anion is weakly bound to the cation and it is hydrolysed forming stronger covalent bonds. Here both elements are ions an anion which has a negative charge and a cation which has a positive charge.
The heaviest of the stable halogens it exists as a semi-lustrous non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius and boils to a violet gas at 184 degrees CelsiusThe element was discovered by the French chemist Bernard Courtois in 1811 and was named two.

. A diatomic compound or diatomic molecule contains two atoms which may or may not be the same. The binary compound list is mentioned in the table below. Unlike ionic compounds they do not dissolve in water nor do they conduct electricity.
The other oxygen compounds are also unstable in water. Therefore strong bases are named following the rules for naming ionic compounds. 2KO 2 2H 2 O 2KOH H 2 O 2 O 2 Li 2 O H 2 O 2LiOH.
Iodine is a chemical element with the symbol I and atomic number 53. It correlates the chemical profile and the bioactivity pattern of plant extracts to guide the isolation of compounds and to identify new or already known bioactive constituents at an early stage dereplication Tawfike et al 2013. They are very hard somewhat brittle solids with extremely high melting points higher than 1000 C or 1800 F.
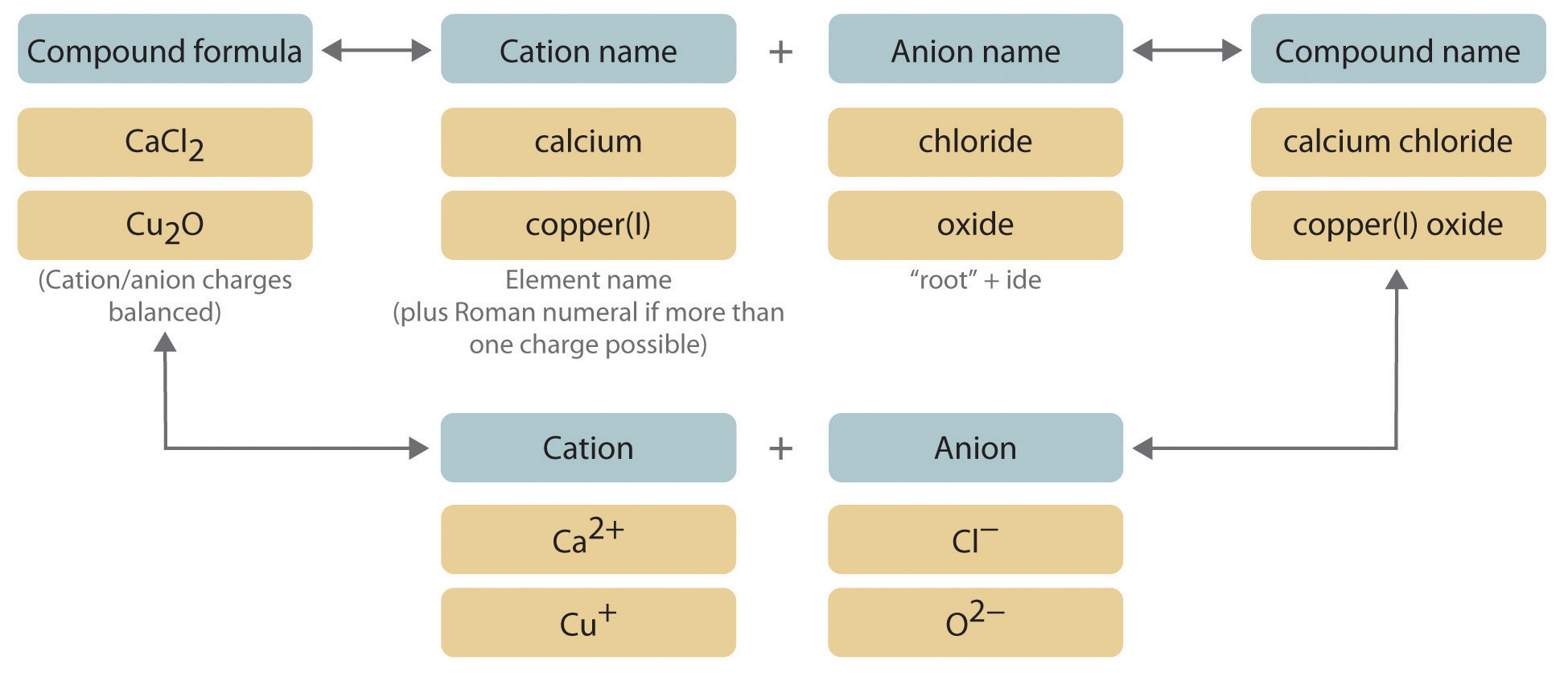
Weak bases made of ionic compounds are also named using the ionic naming system. Binary Ionic Compounds Containing a Metal and a Nonmetal. A binary compound is a compound formed from two different elements.
For example NaOH is sodium hydroxide KOH is potassium hydroxide and CaOH 2 is calcium hydroxide. The goal of metabolomics in general is to analyze all secondary metabolites in a. Nomenclature of Ionic and Covalent Compounds.
Na 2 O 2 2H 2 O 2NaOH H 2 O 2. It was noted that the composition of dispersed phase particles in a liquid dispersion medium should necessarily include adsorbed counterions rigidly bound to these particles. Most strong bases contain hydroxide a polyatomic ion.
Due to the presence of oppositely charged ions ionic compounds are held strongly by the electrostatic force of attraction. The bond formed between them is known as the ionic bond. The alkali metal peroxides are ionic compounds that are unstable in water.
By numerical solution of the Poisson equation for the two most often used approximations the PoissonBoltzmann. From the above example ionic compounds can be defined as the compounds formed by the transfer of electrons between metals and non-metals. The existing concepts of the ionic micelle structure were specified.
There may or may not be more than one of each element. Wolfender et al 2014. Binary ionic compounds are salts which consist of only 2 elements.

Solved Part Ii Binary Ionic Compounds Containing Chegg Com

Naming Ionic Compounds A Guided Inquiry Exercise

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download

Chemistry 101 Naming Binary Ionic Compounds Youtube

Naming Binary Ionic Compounds Rules Examples Expii
5 7 Naming Ionic Compounds Chemistry Libretexts
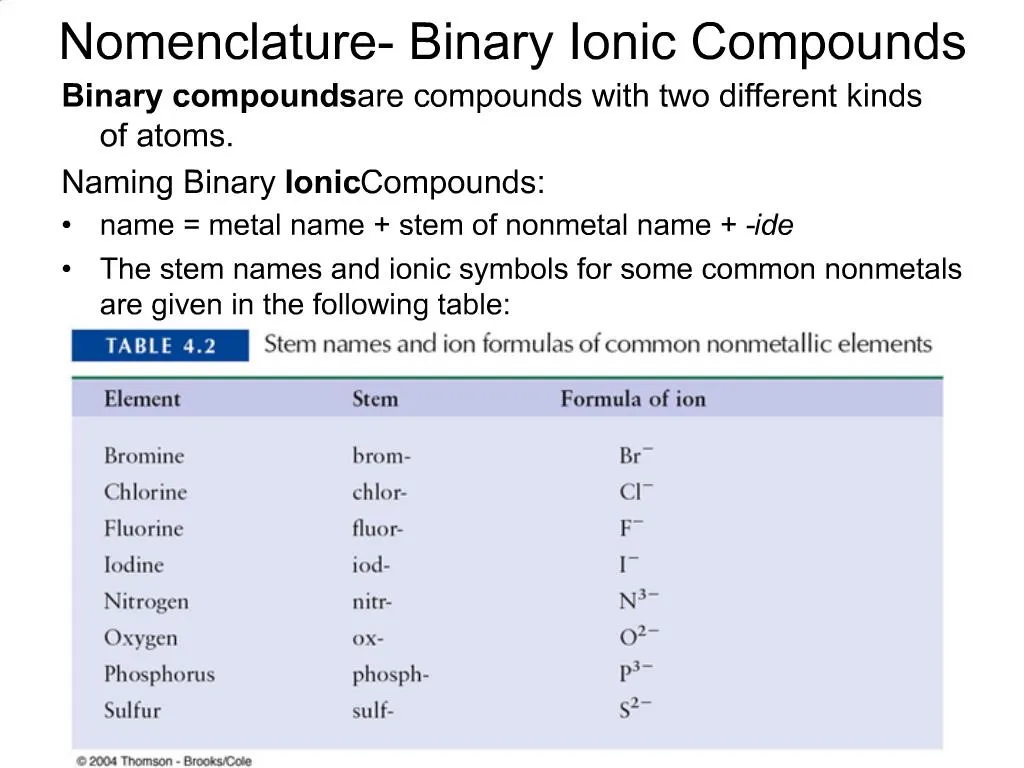
Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132

0 comments
Post a Comment